
Joshi) ozoniser which provided an absorbing column of about 46 cms. The spectra absorbed and emitted by iodine in its atomic and molecular states have been the object of many investigations. Calculate the bond length and B" (rotational constant) of the iodine molecule. The absorption spectrum of iodine was studied using a special design (due to Prof.Assign all the peaks to the 11←5 and 6←3 transitions.A number of band heads were identified and these measurements. An evacuated glass envelope containing a small amount of iodine was used as the absorption cell while a tungsten filament lamp served as the continuous source. The experiment allows one to determine the vibrational constant for an electro.
#Iodine absorption spectrum how to#
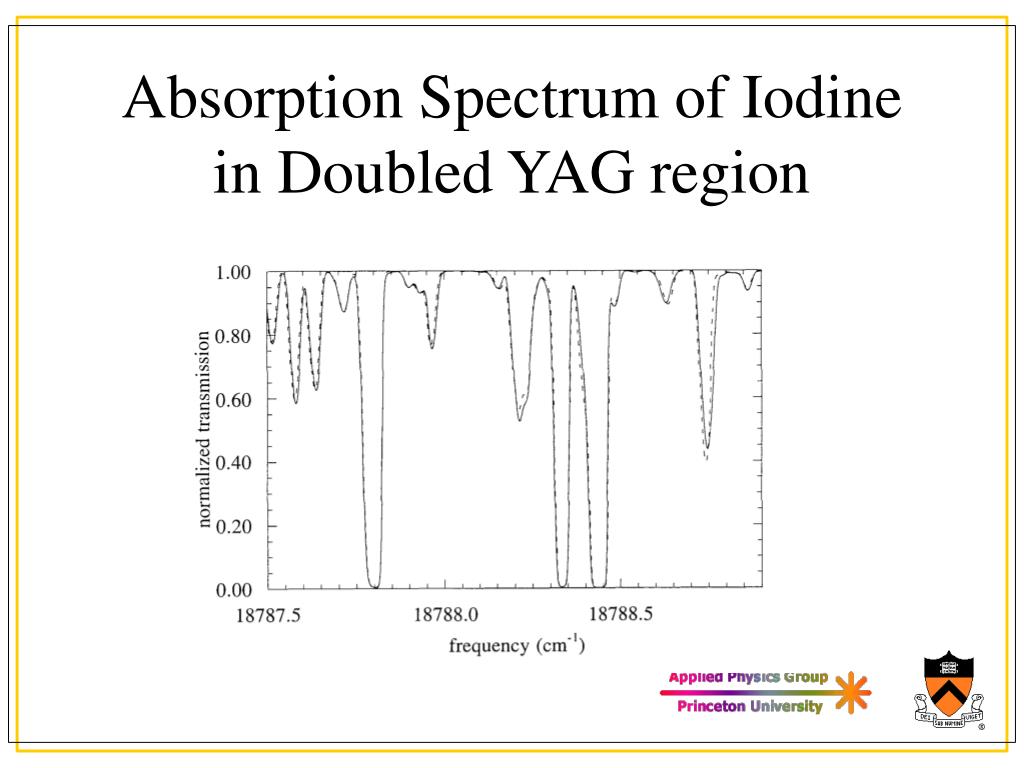
Produce short scans of different Δv lines, especially for those that originate from v'=11, where the separation between transitions to J"=127 and J"=129 can be easily resolved. The absorption spectrum of iodine was photographed in the visible region using a 3.4m Ebert planegrating spectrograph. Shows how to prepare a sample and take a UV-Vis spectrum of iodine (I2) vapor. Heat the fluorescence cell up to ∼80☌. From these data and the data obtained from the B state, calculate the energy of excitation of the iodine atom and compare with literature values. Calculate the values of $ \tilde''_e x''_e $, D'' 0 and D'' e and compare with literature data. You can also use the facts that the v"=0 to v'=27 transition is located at 541.2 nm, and v"=0 to v'=29 transition is located at 536.9 nm. You can use the results of that work for correct assignment. This is the README from the set of files used to run Forkeys iodine absorption spectrum code. It is interesting to note that although the iodine spectrum was first measured in the beginning of the 20th century, the correct v' assignment was reported only in 1965 by Steinfeld et al. Correctly assigning the v' numbers to the different absorption peaks can be difficult, because the v"=0 to v'=0 transition is too weak to be observable, and therefore the numbers cannot be obtained by simple counting. Curves indicate use of band intensities to identify lower state vibrational quantum numbers. Plot of intensity versus wavelength for molecular iodine. Figure 1 shows how the changing intensities can be used to identify the peaks that arise from the v"=0 band. They can, however, cause some confusion in deciding which bands arise from the ground state. These shoulders are "hot" bands arising from the v"=1 and v"=2 to v' transitions and will not be considered in this experiment. At wavelength longer than about 550 nm, some "shoulders" appear on the absorption bands arising from the v"=0 to v' transition. In the diagram below, you can see the absorption spectra of three key pigments in photosynthesis: chlorophyll a, chlorophyll b, and -carotene. Introduction: The spectrum of molecular iodine was among. The set of wavelengths absorbed by a pigment is its absorption spectrum. Between 500 nm and 550 nm, all observed transitions originate in the state v"=0. cm-1) were large because of the propagated error originating from the slopes of the BirgeSponer plots. Tabulate the experimental values of the absorption maxima in nm and cm-1. Do not forget to make corrections to the wavelength based on the results of the holmium oxide calibration. Measure the spectrum at intervals of 5☌ from room temperature to 40☌. Table of contents No headers Given that the 0 to 25 transition occurs at 545.5 nm, assign the peaks in the spectrum according to their values. Use a wavelength range from 500 nm to 600 nm. Measure the absorption spectrum of the iodine gas with the highest resolution possible. Align your setup so that 500 μm slits produce an intensity of 1000. Assemble the setup of the lamp and two lenses. Calibrate the monochromator using a known emission spectrum (hydrogen or mercury). The emission spectrum of iodine has been photographed in the visible region at high resolution, and 16 bands of the B 0 + u ( 3 ) X 0 + g ( 1 ) system have been rotationall analyzed. 430-432).In this part of the experiment, you will study the vibrational structure of the iodine molecule by measuring its absorption spectrum. Compare all of your results with literature values (See, for example, reference. Save a copy of this graph (or take a screenshot) for your ELN. 
The MDL was determined to be 0. The two Morse potentials will be plotted on one graph and the vibrational quantum levels will be shown for both ground and excited states. It was found that the highest spectrophotometry signal for the same starch concentration occurs at pH 6.0, with an iodine reagent concentration of 0.2.






 0 kommentar(er)
0 kommentar(er)
